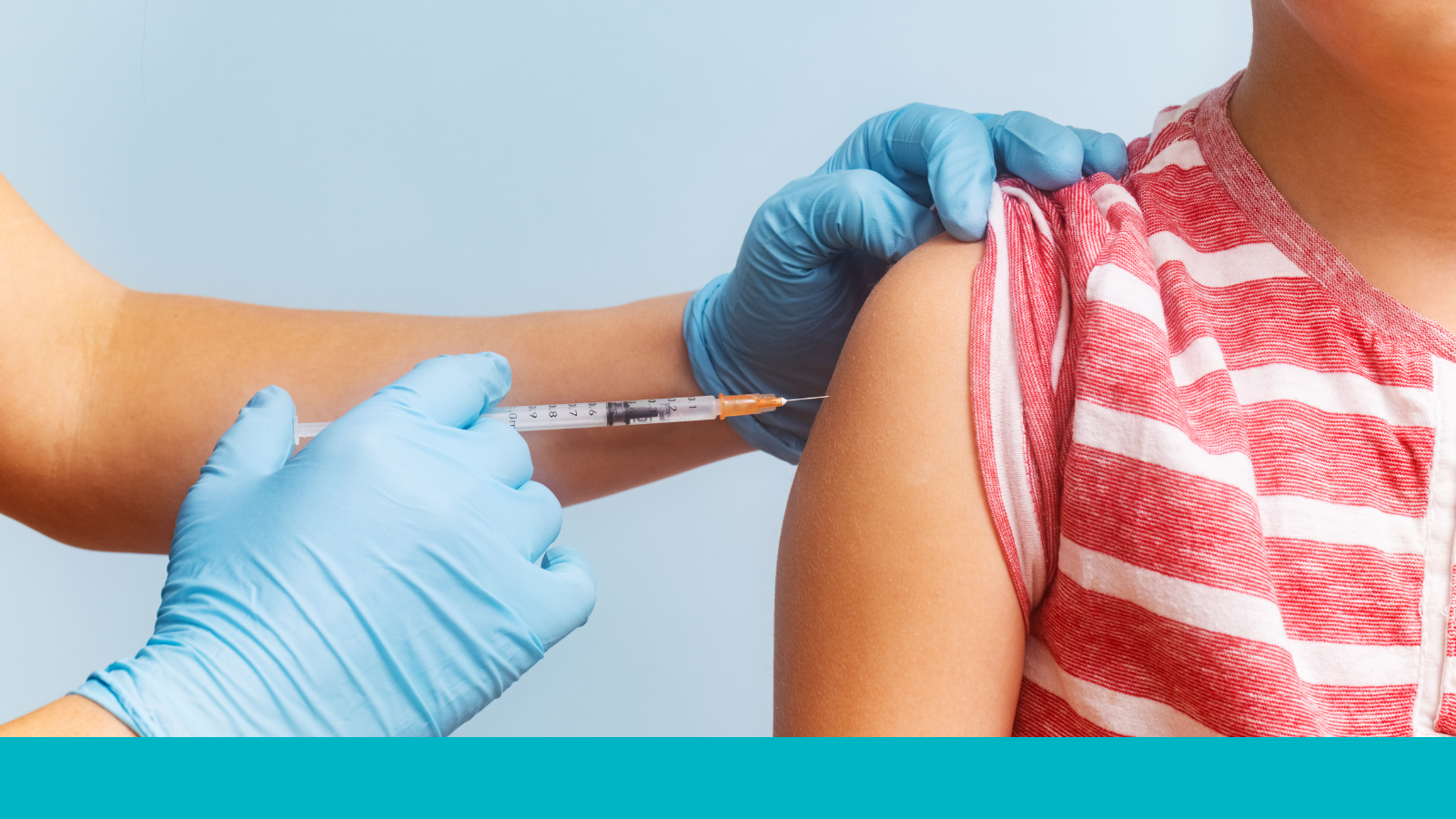
Vaccination is the most effective way to avoid contracting measles and can also help limit its spread in communities. Within two to three weeks of receiving the recommended doses of the measles-mumps-rubella (MMR) vaccine, the immune system is ready to protect against measles. In rare cases, fully vaccinated people may contract measles, but their symptoms tend to be mild compared to those who are unvaccinated and contract measles.
Last updated: November 4, 2025
Options
- Two vaccines can help prevent measles in children ages 12 months to 12 years old: the measles-mumps-rubella (MMR) vaccine and the measles-mumps-rubella-varicella (MMRV) vaccine.
- The MMR vaccine protects against measles, mumps, and rubella.
- The MMRV vaccine protects against measles, mumps, rubella, and varicella, also known as chickenpox.
Doses
- Both the MMR vaccine and the MMRV vaccine require two doses. The first dose of either vaccine is typically administered between ages 12 and 15 months. The second dose of either vaccine is typically administered at 4 years old.
- Since 2009, the Centers for Disease Control and Prevention (CDC) has recommended that the first dose be the MMR vaccine with a separately administered first dose of the varicella vaccine, though the combined MMRV vaccine remains available as a first dose option if preferred by a parent or caregiver and approved by a health care professional. The CDC recommends either the MMR, with a separately administered varicella vaccine, or MMRV vaccine for the second dose.
- The MMR and MMRV vaccines are combination vaccines, which means they protect against several diseases in a single dose to reduce the number of injections a person receives and the number of visits to a healthcare professional. There are no separate vaccines to protect against measles, rubella, or mumps only.
Getting the Vaccine
- The American Academy of Pediatrics (AAP) schedule of recommended vaccines is the most effective way to protect infants, children, and teens against measles. The AAP schedule recommends giving families a choice of the MMR or MMRV vaccine for the first dose.
- The AAP recommendation differs from the Advisory Committee on Immunization Practices (ACIP) recommendation, which influences the CDC’s guidance. In September 2025, ACIP recommended, without scientific evidence, that the MMRV vaccine option should not be used for the first dose. Instead, ACIP recommends the first dose of the MMR vaccine at ages 12 to 15 months with a separately administered varicella vaccine, followed by a second dose, which can be the combined MMRV vaccine at the age of 4.
- The stated reason for this change in recommendation is to help further reduce an already minimal risk of seizures, also known as febrile seizures, that may occur after receiving the first dose of the MMRV vaccine. With the first dose, there is a slightly higher risk of a seizure after receiving the MMRV vaccine compared to the MMR vaccine.